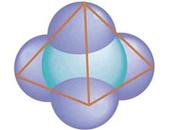
Carbon atoms can bond to up to four other atoms. The four neighboring atoms
of each carbon atom form a tetrahedron, i.e., a pyramid-shaped polyhedron.
This was a key scientific discovery in the late 19th century. Several carbon
atoms can bond to each other to form chains and branches in many different combinations, sometimes yielding extremely long molecular chains. The building blocks of such polymers are small molecules (monomers).
To reach today’s level of knowledge science has come a long, circuitous way.
In ancient Greece experiments were frowned upon. The scientists of those
days – philosophers – believed that insight was to be gained exclusively through observation and rationalization. Around 400 B. C., Democrite of Abdera
postulated, in accordance with his teacher Leukipp, that all of nature is composed
of different smallest, indivisible units he called atoms. In opposition to this,
some 50 years later Aristotle came to the conclusion that all matter consists of varying combinations of merely four elements: air, earth, fire and water, which themselves are composed of a ”quintessence“ called ether. The philosophers of ancient china categorized the world according to dark/strong (male) and
sunny/weak (female) principles called ”Yin“ and ”Yang“, with the vital energy
“Chi” as a corresponding principle to the Greek ether. In Indian Ayurvedics, man
and nature were classified by the terms ”Pittha“, ”Vatha“ and ”Kappha“, and
“Prana” was the life-energy. All of the traditional Greek, Indian and Chinese
views are still popular today in alternative medicine.
The terms ”atoms“ and ”elements“ were coined in ancient times but are still in use today, although now
with modern scientific meanings.
But let’s get back to the roots of chemistry: Despite all their extensive
experiments, the alchemists of the middle ages never succeeded in turning base metals into gold. However, they discovered a number of useful manufacturing techniques, e.g., porcelaine. In the late 18th century, chemists began to analyze known substances and to synthesize new ones systematically. In the early 19th century, the English chemist John Dalton concluded from his observance that
reaction stoichiometries followed distinct proportions that the underlying reason
for this must be the existence of atoms. To top it off, Dalton derived from his
atomic theory a method of determining the weight of atoms. He declared that
each atomic species can be discerned solely by its atomic weight. In the light of today’s knowledge, the latter is true only to a limited extend, because there are chemically identical atoms of different atomic mass (isotopes) and also different atomic species of the same mass. In the 19th century, the German chemist
Friedrich Wöhler disproved the hitherto held dogma that organic substances can
only be produced in living organisms when he synthesized urea from an inorganic compound.
In the 19th century, a number of important technical inventions were made, all harkening back to the treating of natural substances with chemical agents under
high pressure and temperatures. In 1839, the American Charles Goodyear found
a method of hardening natural rubber (caoutchouc), a soft elastic substance,
which was used mainly for erasing at that time and which is made from latex,
i.e., the sap of rubber trees. Goodyear called his technique ”vulcanizing“, because this term implied the treatment of caoutchouc with heat and sulfur. Vulcanizing
turns natural rubber into a dry, tough, yet elastic material well suited for making
car tires. Thus Goodyear’s invention was to soon revolutionize transportation.
A little later, in 1856, the Englishman William Henry Perkin unwittingly produced mauveine, the first synthetic dye, while he was trying to make chinine from
aniline (a tar derivative). This fluke was to initiate intense research worldwide
which lead to a multitude of further dyes and – eventually – to pharmaceuticals,
too.
Simultaneously, a breakthrough was made in fundamental chemical research which was to later lay the foundation of polymer science. In 1858, the German chemist Friedrich Kekulé proved that a carbon atom can only form chemical
bonds to up to four other atoms. He also found that carbon atoms can link to each other thus forming chains and rings. Nearly at he same time, these findings were also made by the Scottish chemist Archibald S. Couper. In 1874, both the
Dutchman Jacobus van’t Hoff and the Frenchman Josef Le Bel independently suggested the tetrahedron model of carbon, in which the four bonds of carbon
are pointed towards the vertices of an equihedral pyramid. It was not until then
that the steric structure of organic molecules as well as of natural and synthetic polymers could be fully understood.
28.12.2007
Artificial skin has been laboratory-grown on a polymer scaffold and can be used for healing chronic wounds of patients with ulcers caused by insufficient blood flow (Ulcus cruris).
-> More
09.01.2008
Polymerizing Ethylene and Propylene to Polyethylene and Polypropylene.
-> More
19.01.2009
PET, a polyester, is a chemical feedstock in polymer industry. Its synthesis may follow either of two ways ...
-> More