Although both the science and technology of polymers had advanced remarkably
by the early 1950s, some formidable challenges remained to be surmounted. Because of the apparently abundant supply and low cost of their component petroleum-derived building blocks or ”monomers“, hydrocarbon polymers
containing only carbon (C) and hydrogen (H) atoms represented a potentially
highly useful class of substances. Particularly attractive targets were polymers
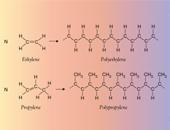
of the smallest and most abundant such monomers, ethylene and propylene (containing two and three carbon atoms, respectively). The general ability of
such molecules, containing pairs of carbon atoms connected by ”double bonds“,
to join together to form long chains (polymers, see figure) had long been
recognized (a familiar example being polystyrene). However, in the case of
ethylene and propylene this presented a formidable challenge. The
polymerization of ethylene had been accomplished, but only at undesirably high temperatures and pressures, yielding polymers whose properties left much to
be desired. The polymerization of propylene remained to be achieved.
In 1953, while engaged in basic research on the reactions of compounds
containing aluminum-carbon bonds, the German chemist Karl Ziegler, working
at the Max Planck Institute for Coal Research in Mulheim, discovered that adding salts of certain other metals such as titanium or zirconium to these compounds resulted in highly active ”catalysts“ (substances that speed up chemical reactions)
for the polymerization of ethylene under relatively mild conditions. Furthermore,
the polymers formed in this way, because the chains were longer and more
linear, had greatly superior properties such as strength, hardness, and chemical inertness, making them very useful for many applications.
Building on Ziegler's discovery, Italian chemist Giulio Natta, working at the Milan Polytechnic Institute, demonstrated that similar catalysts were effective for the polymerization of propylene. Furthermore, with such ”Ziegler-Natta catalysts“, it was possible to achieve exquisite control of the chain length and structures of the resulting polypropylene polymers and, thereby, of their properties. Among other
remarkable achievements of this class of catalysts was the synthesis of a
polymer that is identical to natural rubber.
Industrial applications of ”Ziegler-Natta catalysts“ were realized almost
immediately and with various subsequent refinements continue to expand.
Today, polyethylene produced with such catalysts is the largest volume plastic material and, together with polypropylene, accounts for about half of the U.S.'s current annual 80 billion pound production of plastics and resins. The uses of polyethylene and polypropylene extend to virtually every facet of industry and
daily life, including building and construction materials, containers, toys, sporting goods, electronic appliances, textiles, carpets and medical products. In many of these applications polymers replace other substances, such as glass and metals,
but their distinctive properties also have given rise to entirely new applications, including medical uses.
In 1963, the Nobel Prize in Chemistry was awarded to Ziegler and Natta ”for their discoveries in the field of the chemistry and technology of high polymers“. In his acceptance speech, recalling the circumstances of his pioneering discovery and
the scientific obstacles that had to be overcome, Ziegler went on to say:
”But a much more formidable impediment might have presented itself. In order
to illustrate this, I must elaborate on the paradox that the critical concluding
stages of the investigations I have reported took place in an institute for 'coal research'. When I was called to the Institute for Coal Research in 1943, I was disturbed by the objectives implied in its name. I was afraid I would have to
switch over to the consideration of assigned problems in applied chemistry.
Since ethylene was available in the Ruhr for coke manufacture, the search for
a new polyethylene process, for example, could certainly have represented
such a problem. Today I know for certain, however, and I suspected at the time,
that any attempt to strive for a set goal at the very beginning would have
completely dried up the springs of my creative activity.“
28.12.2007
Artificial skin has been laboratory-grown on a polymer scaffold and can be used for healing chronic wounds of patients with ulcers caused by insufficient blood flow (Ulcus cruris).
-> More
19.01.2009
This timeline highlights the most fundamentally important inventions and
research results leading to a better understanding of polymers and to the
development of innovative polymer applications in medicine.
-> More
19.01.2009
PET, a polyester, is a chemical feedstock in polymer industry. Its synthesis may follow either of two ways ...
-> More
09.01.2008
Bakelite, one of the first plastics, was invented by Leo Baekeland in 1909. Its
rapid success sparked a flurry of synthesis investigations and innovations in both America and Europe.
-> More